Summary Report on Clinical Trial of Home Breast Examining Device
I. Background and purpose of pre-experiment
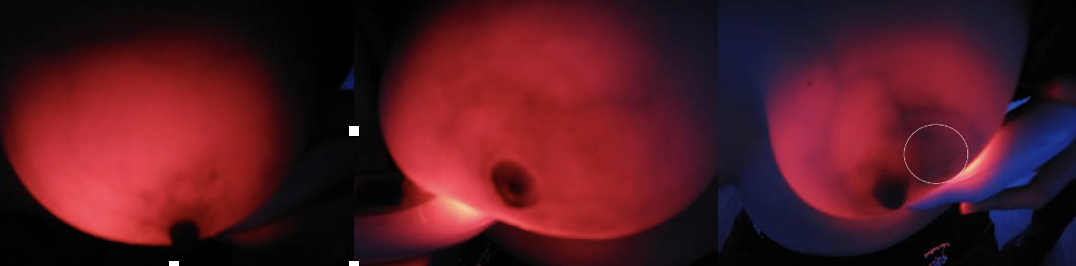
Breast cancer is one of female malignant tumors with high mortality, which seriously threatens women's health. Screening is an effective means to reduce the mortality of breast cancer.Breast near infrared (NIR) screening has been used for more than 80 years.Compared with benign tissue, malignant tumors have more blood vessels, higher blood volume and increased levels of deoxygenated blood.NIR was able to see these changes using a specific wavelength of light (550 to 650nm, the absorption peak of hemoglobin).In recent years, the development of light-emitting diode (LED) technology has enabled the miniaturization of the 625+10 nm near-infrared fluoroscopy device, which can be designed as a hand-held instrument, namely the "breast radiography".The device emits harmless visible red light at that wavelength and appears black where blood vessels are dense (because hemoglobin absorbs the red light), thus indicating a potential malignancy.
The accuracy of mammography as a breast cancer screening method will be evaluated in the future.However, breast fluoroscopy (TBS) is highly dependent on the experience and skills of the operator, and the judgment criteria and demonstration images for various breast diseases have not been unified yet, which may affect the evaluation results of future trials.To this end, the preliminary experiment was carried out to review TBS of clearly diagnosed benign and malignant breast diseases and normal women, summarize the imaging characteristics of various types of breast and obtain typical TBS images, which will be used for TBS training in the future and guide the development of the next evaluation test.
2. Pre-experiment method
1. Selection of experimental subjects and sample size
Six categories of subjects were included: breast cancer (including all clinical stages and types), benign breast fibroma, breast cyst, benign breast hyperplasia, and normal breast women, with 20 cases in each category, 100 cases in total.In addition, 3 patients with mammary gland inflammation were added during the experiment.Inclusion criteria: ① No breast or mass resection;② There are clear imaging or pathological diagnosis results;③ Voluntarily participate in the survey and sign the informed consent.
2. Check procedures and flow charts
(1) Check the environment: Check in the dark room.
(2) inspection procedures :(figure 1)
In the first step, the clinician explained the project to the subjects who met the inclusion criteria, and the subjects entered the examination room after signing the informed consent letter.
The second step was to number the subjects and conduct an epidemiological investigation (including general demographic characteristics, reproductive history, disease history and family history).
The third step was to read the imaging or pathological diagnosis report of the subject. After locating the lesion through observation and palpation, the breast radiography was used to focus on the lesion location, and the TBS imaging features of the lesion were carefully observed, and the image data were collected.Afterward, a thorough examination of both breasts and armpits was performed.
Step 4: Fill in the Evaluation Form of Breast Optical Detector Examination (TBS), and record the TBS imaging characteristics of various breast diseases from the aspects of overall breast transmittance, mass shadow, vascular shadow and the relationship between them.
(3) Examination position: Client is topless, sitting or standing facing the examiner, leaning forward slightly with chest relaxed.
(4) Examination method: The examiner holds the mammary diptometer in his left hand, and the light source is close to the lower part of the breast. The breast is held up and the intensity of the light source is adjusted, and the four parts of the areola, the inner side of the breast, the outer side of the breast and the armpit are observed in order.
……
Original Files Download:
LSC6007CN_Summary Report on Clinical Trial of Home Breast Examining Device
LSC6007EN_Summary Report on Clinical Trial of Home Breast Examining Device