Summary Report on Clinical Trial of Home Breast Examining Device
I. Background and purpose of pre-experiment
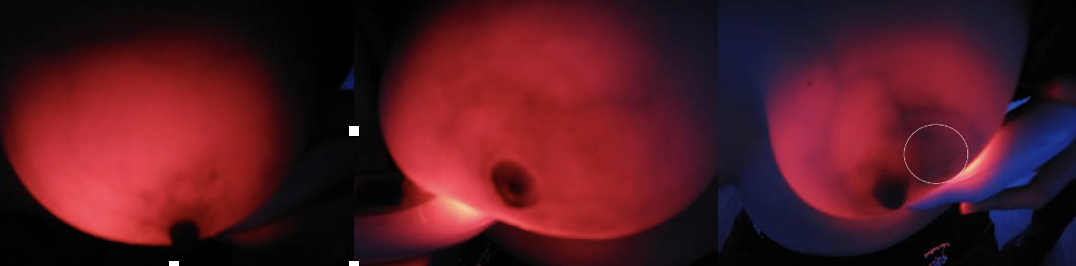
Breast cancer is one of female malignant tumors with high mortality, which seriously threatens women's health. Screening is an effective means to reduce the mortality of breast cancer.Breast near infrared (NIR) screening has been used for more than 80 years.Compared with benign tissue, malignant tumors have more blood vessels, higher blood volume and increased levels of deoxygenated blood.NIR was able to see these changes using a specific wavelength of light (550 to 650nm, the absorption peak of hemoglobin).In recent years, the development of light-emitting diode (LED) technology has enabled the miniaturization of the 625+10 nm near-infrared fluoroscopy device, which can be designed as a hand-held instrument, namely the "breast radiography".The device emits harmless visible red light at that wavelength and appears black where blood vessels are dense (because hemoglobin absorbs the red light), thus indicating a potential malignancy.
The accuracy of mammography as a breast cancer screening method will be evaluated in the future.However, breast fluoroscopy (TBS) is highly dependent on the experience and skills of the operator, and the judgment criteria and demonstration images for various breast diseases have not been unified yet, which may affect the evaluation results of future trials.To this end, the preliminary experiment was carried out to review TBS of clearly diagnosed benign and malignant breast diseases and normal women, summarize the imaging characteristics of various types of breast and obtain typical TBS images, which will be used for TBS training in the future and guide the development of the next evaluation test.
2. Pre-experiment method
1. Selection of experimental subjects and sample size
Six categories of subjects were included: breast cancer (including all clinical stages and types), benign breast fibroma, breast cyst, benign breast hyperplasia, and normal breast women, with 20 cases in each category, 100 cases in total.In addition, 3 patients with mammary gland inflammation were added during the experiment.Inclusion criteria: ① No breast or mass resection;② There are clear imaging or pathological diagnosis results;③ Voluntarily participate in the survey and sign the informed consent.
2. Check procedures and flow charts
(1) Check the environment: Check in the dark room.
(2) inspection procedures :(figure 1)
In the first step, the clinician explained the project to the subjects who met the inclusion criteria, and the subjects entered the examination room after signing the informed consent letter.
The second step was to number the subjects and conduct an epidemiological investigation (including general demographic characteristics, reproductive history, disease history and family history).
The third step was to read the imaging or pathological diagnosis report of the subject. After locating the lesion through observation and palpation, the breast radiography was used to focus on the lesion location, and the TBS imaging features of the lesion were carefully observed, and the image data were collected.Afterward, a thorough examination of both breasts and armpits was performed.
Step 4: Fill in the Evaluation Form of Breast Optical Detector Examination (TBS), and record the TBS imaging characteristics of various breast diseases from the aspects of overall breast transmittance, mass shadow, vascular shadow and the relationship between them.
(3) Examination position: Client is topless, sitting or standing facing the examiner, leaning forward slightly with chest relaxed.
(4) Examination method: The examiner holds the mammary diptometer in his left hand, and the light source is close to the lower part of the breast. The breast is held up and the intensity of the light source is adjusted, and the four parts of the areola, the inner side of the breast, the outer side of the breast and the armpit are observed in order.
……
Original Files Download:
LSC6007CN_Summary Report on Clinical Trial of Home Breast Examining Device
LSC6007EN_Summary Report on Clinical Trial of Home Breast Examining Device
Construction Plan of Elemage Medical Devices Manufacturing Site
Project name: Construction of portable medical electronic product industrialization base
Project Unit: Beijing Elemage (Shanxi) Technology Co., LTD
Time: Nov. 2010
1. Project Introduction
Beijing Yili Magi Technology Co., Ltd. is a high-tech enterprise registered in Beijing Shangdi Economic Development Zone, mainly committed to the research and development of health-related medical devices, medical materials and other products, the research team is composed of senior technical personnel at home and abroad.The company has a series of product projects under development, so far 5 projects have been approved, and 2 designs and prototype trial production have been completed. According to the project development plan, by the end of 2012, 4 medical electronic products and health related products with the approval number of medical devices will be completed and gradually introduced to the market.In order to speed up the product launch and give full play to the advantages of local funds and policies in Gaoping, Shanxi, the company has decided to establish Beijing Yili Magi (Shanxi) Science and Technology Co., LTD., transplant the project to Gaoping, Shanxi, and establish a production base mainly for producing portable medical electronic products.
According to the overall development plan of the company, the company plans to 8 to 10 years, in both the technology and the production scale and economic benefit etc., have become the industry's leading enterprises and industry guide, become the domestic products industry standard drafting makers, to firmly grasp and guide the development direction of the industry, the formation of ELEMAGE brand series of products, Build a professional company with certain brand benefits, and finally realize the overall listing of the company.
……
Original Files Download:
LSC6006CN_Construction Plan of Elemage Medical Devices Manufacturing Site
LSC6006EN_Construction Plan of Elemage Medical Devices Manufacturing Site
Taihang Pharmaceutical Co., LTD
Taihang Pharmaceutical Co., LTD. (hereinafter referred to as "Taihang Pharmaceutical") is a scientific and technological enterprise which is mainly engaged in the research, production and business of modern Traditional Chinese medicine, and is equipped with the standardized production and processing of the extraction and refinement of the effective ingredients of traditional Chinese medicine.Belonging to the "Zhanghe" brand is a famous trademark in Shanxi Province.Taihang pharmaceutical is one of the top 100 Chinese medicine brand enterprises in China, a national credit investigation enterprise, the national "drug quality integrity Construction demonstration enterprise", one of the leading enterprises in Shanxi Province to revitalize the pharmaceutical industry planning industry, Shanxi Province quality credit AAA level enterprise, "abide by contract and credit" enterprise.
Tibet Pharmaceutical Manufacturing Co., LTD
Members of the investigation team communicated with Mr. Zhang, the site coordinator of the Project of the College of Tibetan Medicine, and Mr. Zhang summarized the current situation, business and workshop production of the College of Tibetan Medicine.Subsequently, the investigation team members were led by Zhang to the headquarters base, planting base and color wheel Tibetan Incense factory of Tibetan Medicine Co., LTD., College of Tibetan Medicine for field investigation.Through this investigation, we have summarized the information of pharmaceutical companies in the College of Tibetan Medicine so far, as follows:
Background of Tibetan Medicine Co., LTD., College of Tibetan Medicine:
The Tibetan Medicine Co., LTD., formerly known as The Tibetan Medicine Factory of Tibet College of Tibetan Medicine, was founded in 1996, located in the northeast of The Potala Palace, covering an area of 10,000 square meters, in the hospital of Tibet College of Tibetan Medicine. In 2008, it was restructured into Tibet College of Tibetan Medicine Co., LTD., with a registered capital of 31.2 million yuan and more than 130 employees. In 2009, the company passed the national GMP certification and ISO9001:2008 certification again……
Original Files Download:
Project Investigation Report on Tibet Pharmaceutical of Tibet Medical Institute
Due to insufficient investigation time, materials and data to write a standard due diligence report, we had to write a project investigation report for the reference of investors.
After communication with the person in charge of the company and field visit to the production plant in Lhasa and the marketing center in Chengdu, the overall feeling of investing in the project is "it is a pity to abandon, but it is also a risk to invest".
The so-called risk refers to the comprehensive investment risk brought by the existing production conditions and hardware level, market, management and the current high valuation.
……
Original Files Download:
Aidphar Business Plan (2001)
Abstract
Company's business model:
Our company is a start-up company.Shanxi Aidphar pharmaceutical Co., Ltd. legal management form is limited liability company, legal address: Jincheng Economic and technological Development Zone. Production address: Jincheng Economic and technological Development Zone.
In recent years (1998-2001), our company researchers have made achievements in the development of drugs for children, embodied in: 1, fill the domestic blank of cold medicines for children - Ed Koch son was on February 10, 2001 in the national new drug evaluation committee by pharmacology, pathology, toxicology, clinical evaluation, is listed in the near future.2. Children's special immune-enhancing drug -- Aderqiang has started project development.
In order to popularize the above two new drugs to the society and benefit human health.And continue to develop a series of new drugs for children research and development, we officially registered in October 2000 Shanxi Aid pharmaceutical Co., LTD.We are determined to introduce a series of new children's medicines to the market, create the expected social and economic benefits, achieve our cause, achieve the purpose of the enterprise, and make unremitting efforts for the cause of human health.
Enterprise's business field and mission
1.Enterprise positioning: committed to children's health cause, using advanced technology to provide customers with better products and services;To provide a platform for employees to realize their personal ideals;Create more wealth for society and shareholders.
Create health for customers
Create opportunities for employees
Create wealth for society
Objective and task: healthy enterprise structure -- [Science, industry, trade, resources]
Within a certain strategic period, our goal --
2001-2005 - Image of child health expert recognized by the public in China
-- Leading position in children's medicine in China;
2006-2010 -- Domestic Children's Health (pharmaceutical) scientific Research Base (virtual)
-- Sinopharm children first dose.
3.Business philosophy
(1) Business philosophy: customer is stream, market is sea;
Capital is the ship, brand is the sail;
Talent is the foundation and culture is the soul.
(2) Business purpose: to be a man and make medicine;Offer love and cultivate morality.
(3) Business policy: customer-oriented;
Market-oriented;
Creative guidance;
Meet market demand.
Company background
1.Aidphar pharmaceutical Co., Ltd. is a new high-tech pharmaceutical enterprises, registered capital of 5 million yuan, is located in Jincheng Economic and technological Development zone, Shanxi Province, superior environment, convenient transportation, is in jincheng economic restructuring in the context of the emergence of the first batch of model enterprises.At the beginning of its establishment, the company has established the road of sustainable development of "committed to children's health", and established the enterprise purpose of "life, medicine, love and virtues" and the brand concept of "sincere love, care for the future".Since its establishment, it has been attached importance and care to provincial and municipal leaders and competent departments, and can enjoy preferential policies that similar enterprises can enjoy in the development zone.
2.At present, we have completed new product production report, enterprise strategy and human resources, finance, marketing and other related planning, plant planning and layout in line with GMP certification, earthworks.The organization system is being set up and improved, and the promotion plan, capital operation and related work are also being carried out as scheduled.
Financial overview
From the perspective of expected financial analysis, we are expected to reach 98 million yuan in sales revenue and 28.73 million yuan in after-tax profit in 2004.In 2005, sales revenue reached 133 million yuan and after-tax profit reached 36.03 million yuan (see below for recent and accurate financial analysis).The reason why we can achieve this goal is that we have a complete and scientific capital operation plan, the capital is mainly used for the construction and purchase of GMP plant equipment;Capital for laying the foundation for production.
Product status
The company's current product status is: eight generic drugs, two new drugs - Aderkam (expected to be launched in October 2001), aderkam (under development, expected to be launched in late 2002 or early 2003).Ed Koch, including from the United States the introduction of a new generation of infantile cold medicines, belong to national three kinds of new drugs, drug ingredients is internationally recognized as good curative effect, less toxic antipyretic analgesic cold medicines, more important is to choose the children's medicine optimal dosage forms - dispersible tablets, instant see water, fruit fragrance, completely solve the other dosage forms for children, especially infants and young children caused by the medicine of the difficult problems and dose not accurate.
Market capacity - the total market capacity of cold medicine for children is 1.5 billion to 2 billion yuan per year
The market localization
1.options
Jews don't have a proper but business quotes is worthy of learning "good money" women and children, at the beginning of the enterprise product project, we have to existing drugs market completely to comb again, found "a pharmaceutical manufacturer in industry, we summed up in four words, namely" the old man to prolong life, man to kidney strong sun, women want to lose weight and beauty, children to raise Gao Jian brain ", in the intellectual and professional market research company, with the help of our one ruled out the beginning should not enter into the old man of narcotic drugs, men, women, drug market, and choose the children's drugs market.
2.The market localization
Targeted at children's drugs market, we are not eager to develop products, but the vision will be turned to the children's drugs market research, because love is a new pharmaceutical enterprise, at the start-up stage, must look before you leap, through comprehensive analysis, we chose the children's drugs capacity larger cold medicine market, choose good product after it will be the ultimate goal of the public market positioning in 0 ~ 12 years old children.
3.Target the public and customer groups
Because of the particularity of children's drugs, the consumer and the buyer's for complete separation, the decision-making process is more complicated, we target the public seems to 0 ~ 12 years old children, in fact demands is the goal of public parents, especially his mother, they buy standard is "safe, effective, brand, convenience, price", to be able to continue to maintain the purchasing power of this part of the group can only rely on safe, effective, and convenient to maintain, at the same time to guide its brand image for the establishment of brand choice and loyalty.
competition
1.Industry status
With the development of market economy and the continuous maturity of the system, each industry is full of competition. Although the children's drug market is our careful choice, the competition in this industry is still more intense (but compared with many other adult human pharmaceutical products market, the competitive environment is relatively loose).In terms of the current domestic pharmaceutical market for children, Johnson & Johnson and Shanghai Squibb are in the first group, followed by Nanjing Chengong Zexin and Beijing Sunde Pharmaceutical.
2.Competition of cold medicine for children
The main products are: Tylenol, Baifu one, children cold infusion;
The main problems are :(1) adult medication;(2) Inconvenient to take;(3) The dosage is not correct.
3.The advantage of Adercom
This product selects the most advanced dosage form of children's medicine -- dispersive tablet, which fundamentally solves the inconveniences of children's medicine. In a certain sense, dispersive tablet is the key development dosage form of children's medicine market in the future.
Original Files Download: